Flacons de médicaments biologiques – EVERIC® pure Des flacons pour les médicaments biologiques et une stabilité des médicaments inégalée
Obtenir un devisStabilité accrue des médicaments et contrôle de la délamination pour les faibles volumes de remplissage aseptique
L'augmentation du nombre de produits biologiques oblige les fabricants de systèmes de confinement à s'adapter aux besoins de ces médicaments sensibles.
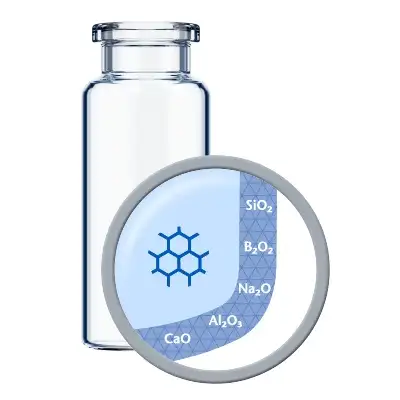
Les flacons de médicaments biologiques standard présentent souvent des niveaux de lixiviation plus élevés pour les faibles volumes de remplissage, ainsi qu'un risque accru de changement de pH et de délamination. Ces problèmes sont dus à une modification de la surface intérieure de la zone proche du fond du flacon lors du processus conventionnel de conversion des flacons.
La réponse à ces défis est EVERIC® pure. Grâce à une technologie de fabrication brevetée, ces flacons de médicaments biologiques présentent une surface intérieure homogène. L'amélioration du comportement de lixiviation rend ce flacon indispensable pour les besoins de stabilité des médicaments biologiques.
Flacons de médicaments biologiques pour un remplissage aseptique
Les flacons EVERIC® pure sont idéaux pour les médicaments biologiques et à faible volume de remplissage en raison de leur stabilité éprouvée.
Les flacons EVERIC® pure offrent également une grande stabilité pour les formulations stérilisées en phase terminale, telles que les diluants, qui nécessitent un stockage spécifique.
De plus, les flacons EVERIC® pure sont idéalement adaptés aux applications d'eau pour injection (WFI) en raison de leur faible niveau de lixiviation et de leur capacité à éviter le changement de pH.
SCHOTT Everic® pure – L'étalon-or de la stabilité des médicaments biologiques
La large gamme d'avantages des flacons EVERIC® pure reflète nos processus de fabrication dédiés.
- Un faible niveau de lixiviation est particulièrement adapté aux médicaments à faible taux de remplissage.
- Le processus de conversion breveté de SCHOTT Pharma garantit un contrôle total de la délamination, ce qui ne compromet pas l'intégrité du médicament.
- Les flacons EVERIC® pure offrent un changement de pH et une conductivité plus faible, ce qui garantit la stabilité du produit.
- La composition inchangée du verre signifie qu'aucun réenregistrement n'est nécessaire.
Les flacons EVERIC® pure – un portefeuille solide pour vos médicaments à faible volume de remplissage
- L'exceptionnel tube en verre FIOLAX® CHR est utilisé pour tous les flacons EVERIC® pure pour un remplissage aseptique.
- Nous proposons une large gamme de formats (2-50R) afin de fournir la solution parfaite pour tous les types de médicaments.
- Les flacons EVERIC® pure sont disponibles dans un emballage de plateau en vrac ou dans un emballage standardisé prêt à l'emploi adaptiQ®.
- Les flacons EVERIC® pure ont un niveau de qualité TopLine, avec un test statistique de libération en production ("Quicktest") effectué pour contrôler un faible niveau de lixiviation dans la zone du talon.
EVERIC® pure prête à l'emploi
Grâce à l'emballage secondaire hautement standardisé adaptiQ®, les flacons EVERIC® pure sont disponibles dans une configuration prête à l'emploi (RTU) pré-lavée et pré-stérilisée.
Les petits lots de médicaments personnalisés et de produits pharmaceutiques de grande valeur représentent souvent un défi pour les opérations Fill-and-Finish. Ils nécessitent non seulement des contenants de meilleure qualité, mais aussi une plus grande flexibilité, avec des changements fréquents entre les différents médicaments afin d'éviter des déchets coûteux.
Les flacons adaptiQ® peuvent être traités sur une variété de lignes Fill-and-Finish nouvelles et existantes, en gardant les flacons emboîtés tout au long du processus, y compris la lyophilisation.