EVERIC® pure
Your first choice for pharmaceutical glass vials
When it comes to protecting your drug formulations, conventional glass vials often fall short, leading to increased leachable levels, pH shifts, and the risk of delamination.
EVERIC® pure is designed to address these challenges head-on. Combining advanced glass tubing with enhanced hydrolytic resistance and our patented manufacturing technology, EVERIC® pure offers a uniform inner surface that significantly reduces the risk of delamination. Every vial undergoes rigorous testing for leachables throughout the production process, providing you with a reliable container that helps prevent costly reformulations and ensures the stability of your medication.
Choose EVERIC® pure as your trusted vial for safeguarding almost all drug formulations. With EVERIC® pure, you gain peace of mind, knowing that your products are stored in a container that prioritizes quality and consistency from the inside out.
The best thing is, that switching to EVERIC® pure is seamless, with no need for re-registration if you are already using Type I glass vials. This means you can upgrade to a superior solution without disruption.
Reliable choice from the start
EVERIC® pure is the gold standard for Type I Borosilicate Glass vials, offering unmatched patient safety, proven stability, and seamless integration.
EVERIC® pure vials – consistent quality from materials to manufacturing
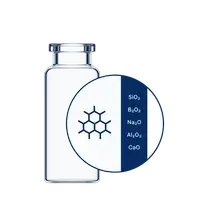
Crafted from pure Type I Borosilicate Glass, EVERIC® pure vials are engineered to meet the rigorous demands of high-value drug development, including biologics. The optimized inner surface enhances formulation stability, providing a broader range of options while minimizing the risk of time-consuming reformulations.
With consistently low leachable levels and reduced pH shifts, EVERIC® pure vials ensure exceptional stability for terminally sterilized formulations, such as water for injection (WFI) and sodium chloride (NaCl). Backed by USP <1660> compliance, EVERIC® pure is the ideal choice to maintain the integrity of applications in phosphate buffers and diluents, effectively mitigating the risk of delamination.

|

|
|
|

|

|
|
|
|

|
|
|
| Product Name |
Product Name
EVERIC® pure
|
Product Name
EVERIC® care
|
Product Name
EVERIC® freeze
|
Product Name
EVERIC® strong & smooth
|
Product Name
EVERIC® smart
|
Product Name
EVERIC® plus
|
Product Name
EVERIC® lyo
|
Product Name
EVERIC® lyo & amber
|
Product Name
EVERIC® amber
|
Product Name
adaptiQ®
|
Product Name
Core Vials
|
| Applications |
Applications
Default solution for biologics, diluents, low-filled and high-value drugs
|
Applications
Drugs in high alkaline pH range
|
Applications
Drugs that need deep-cold storage down to -80°
|
Applications
Improved bulk fill-and-finish
|
Applications
Track and trace solution
|
Applications
Leachable-sensitive drugs in neutral and acidic pH range
|
Applications
Drugs that need lyophilization
|
Applications
Light sensitive, lyophilized drugs, esp. biologics as ADCs
|
Applications
Light-sensitive drugs, esp. biologics as ADCs
|
Applications
Pre-washed and pre-sterilized for leaner fill-and-finish
|
Applications
Default solution for generics, chemicals
|
| Tubing Container Material |
Tubing Container Material
FIOLAX® CHR (controlled hydrolytic resistance)
|
Tubing Container Material
FIOLAX® CHR (controlled hydrolytic resistance)
|
Tubing Container Material
FIOLAX® OS (optimized strength)
|
Tubing Container Material
FIOLAX® OS (optimized strength)
|
Tubing Container Material
FIOLAX®
|
Tubing Container Material
FIOLAX®
|
Tubing Container Material
FIOLAX®
|
Tubing Container Material
FIOLAX® amber
|
Tubing Container Material
FIOLAX® amber
|
Tubing Container Material
Depending on bulk vial used
|
Tubing Container Material
FIOLAX®
|
| Format |
Format
2-50R
|
Format
2R, 6R, 10R
|
Format
2-30R
|
Format
2-30R
|
Format
2-30R
|
Format
2-50R
|
Format
2-50R
|
Format
6R, 10R, 20 ml
|
Format
2R - 10R, 20 ml
|
Format
2-50R
|
Format
2-100R
|
| Quality Level |
Quality Level
TopLine
|
Quality Level
TopLine
|
Quality Level
TopLine
|
Quality Level
TopLine
|
Quality Level
TopLine
|
Quality Level
TopLine
|
Quality Level
TopLine
|
Quality Level
StandardLine
|
Quality Level
StandardLine
|
Quality Level
TopLine
|
Quality Level
StandardLine, TopLine, TopLine Packages
|
| Closure Systems |
Closure Systems
Stopper + seal
|
Closure Systems
Stopper + seal
|
Closure Systems
Stopper + seal
|
Closure Systems
Stopper + seal
|
Closure Systems
Stopper + seal
|
Closure Systems
Stopper + seal
|
Closure Systems
Stopper + seal
|
Closure Systems
Stopper + seal
|
Closure Systems
Stopper + seal
|
Closure Systems
Stopper + seal or press-fit cap
|
Closure Systems
Stopper + seal
|
| Pre-washed Pre-sterilized |
Pre-washed Pre-sterilized
Non-sterile and sterile (adaptiQ®) available
|
Pre-washed Pre-sterilized
Non-sterile and sterile (adaptiQ®) available
|
Pre-washed Pre-sterilized
Non-sterile available
|
Pre-washed Pre-sterilized
Non-sterile available
|
Pre-washed Pre-sterilized
Non-sterile and sterile (adaptiQ®) available
|
Pre-washed Pre-sterilized
Non-sterile and sterile (adaptiQ®) available
|
Pre-washed Pre-sterilized
Non-sterile and sterile (adaptiQ®) available
|
Pre-washed Pre-sterilized
Non-sterile and sterile* (adaptiQ®) available
|
Pre-washed Pre-sterilized
Non-sterile and sterile* (adaptiQ®) available
|
Pre-washed Pre-sterilized
Sterile packaging
|
Pre-washed Pre-sterilized
Non-sterile and sterile (only TopLine in adaptiQ®) available
|
| Packaging | Packaging | Packaging | Packaging | Packaging | Packaging | Packaging | Packaging | Packaging | Packaging | Packaging | Packaging |
| Read More | Read More | Read More | Read More | Read More | Read More | Read More | Read More | Read More | Read More |
*available from Q2 2026