Pharmaceutical track and trace Solutions
The advance of traceability of raw materials, parts, and finished products has been shaped by highly regulated industry sectors whose compliance is legally required, such as the pharmaceutical, aerospace, and automotive sectors. Although traceability has always been a vital aspect of pharmaceutical production, few drug container manufacturers have taken on the challenge of providing traceability along the supply chain. SCHOTT Pharma sets new standards.
Reducing the risk of mix-ups with smart pharmaceutical track and trace solutions for drug containers
Regulations at the national and international level require all pharmaceutical containers to have labels on their secondary packaging. However, current requirements fall short of ensuring full traceability for pharmaceutical products along the entire value chain.
The absence of unique identification on primary packaging containers leads to increased risk and inefficient processes. One example is the risk of mix-ups, typically circumvented by using different colour rings for syringes, or coloured caps for vials. This process, however, has its limits, is inflexible, and prone to error.

Track and trace your drug container from production to the end user
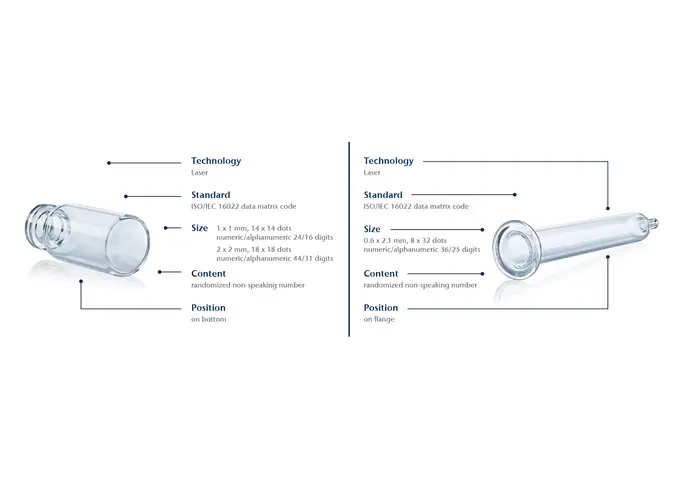
By using a laser to melt a unique data matrix code onto the container the moment it is manufactured, SCHOTT Pharma containers are permanently labelled. The code is inextricably linked to the container at the earliest possible step in the entire value chain.
This offers a great advantages compared with systems wherein the code is only attached at a later stage. The code is also stable during washing, autoclaving and depyrogenation up to a temperature of 600 °C. It resists abrasion, avoiding the risk of particle contamination compared with solutions that require additional substances for the application of the code. The data matrix code can be as small as 1 x 1 mm, which equals 14 x 14 dots.