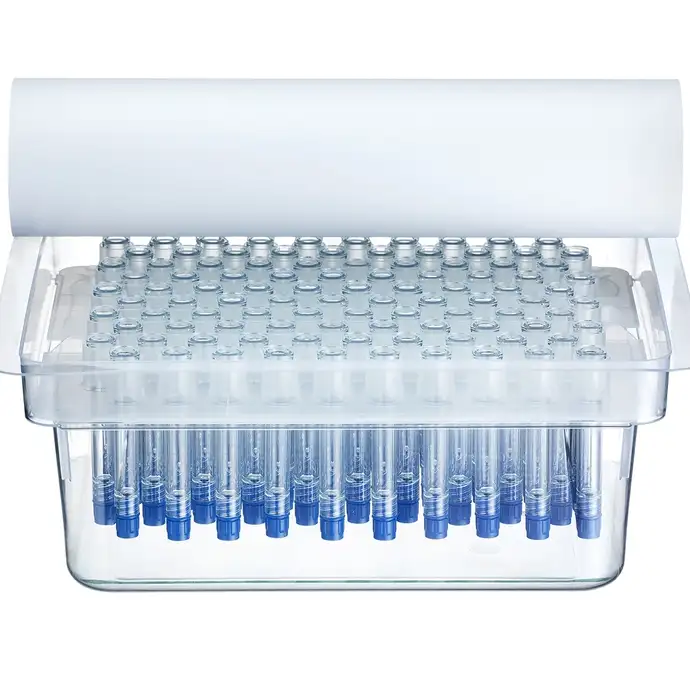
SCHOTT TOPPAC® freeze Ready-to-use prefillable polymer syringes for deep-cold storage drugs
Request quoteA freezing cool solution for deep-cold drugs
Pharma companies understand that when the temperature is low, the requirements for drug containment and delivery are high. The SCHOTT TOPPAC® freeze syringe for deep-cold drugs is ideal for the safe storage and administering of deep-cold medicine. Delivering advanced quality control parameters and an extensive data package on functionality, sterility, CCI, and drug-container interaction, this cutting-edge syringe provides a safe, reliable, and highly effective packaging solution for thawed or frozen medication. Say hello to choice.

Ideal syringe for deep-cold storage
Improved specifications and full data package
A reliable frozen medication storage and delivery solution
- Inert COC material exhibits excellent barrier properties for water vapor and oxygen permeability and results in no ion or metal release.
- COC material manufactured in accordance with EP, JP, and USP class VI (DMF 12132) standards.
- Low protein adsorption, no pH shift, and a low silicone particle burden.
- Available as a 1ml long syringe.
- Supported by application specific drug stability studies (e.g. LNP study or protein stability study).
Available pre-sterilized in a ready-to-use format
This syringe for deep-cold drugs is manufactured in a cleanroom environment using a state-of-the-art, fully automated production process – from injection molding to final packaging in a market standard nest and tub configuration. Using ready-to-use syringes such as SCHOTT TOPPAC® freeze not only enables high-speed filling but also reduces the amount of complex drug-filling processes.