Rethink Pharma manufacturing processes
Contact UsImprove your manufacturing processes with sterile drug containment solutions
Continuous evaluation of pharma manufacturing processes is essential to achieve higher drug yields using less resources. At the same time, however, high-quality standards and flexibility must be maintained. Lower cost, better quality, and higher flexibility can be contradictory goals and require a fundamental rethinking of manufacturing processes.
Three main challenges affect the drug manufacturing and filling process in the pharmaceutical industry:
- Reducing value chain complexity by outsourcing production steps to allow pharma companies to focus on their core business.
- Detecting and eliminating areas of risk along the value chain to ensure patient safety.
- Speeding up time to market to maximize patent lifetimes.
Reduce your value chain complexity and focus on the core
When deciding on a pharma manufacturing process strategy, all costs must be factored in and carefully balanced. Profitability is best assessed by looking at the total cost of ownership (TCO), including all incurring and recurring costs, across the whole project lifetime.
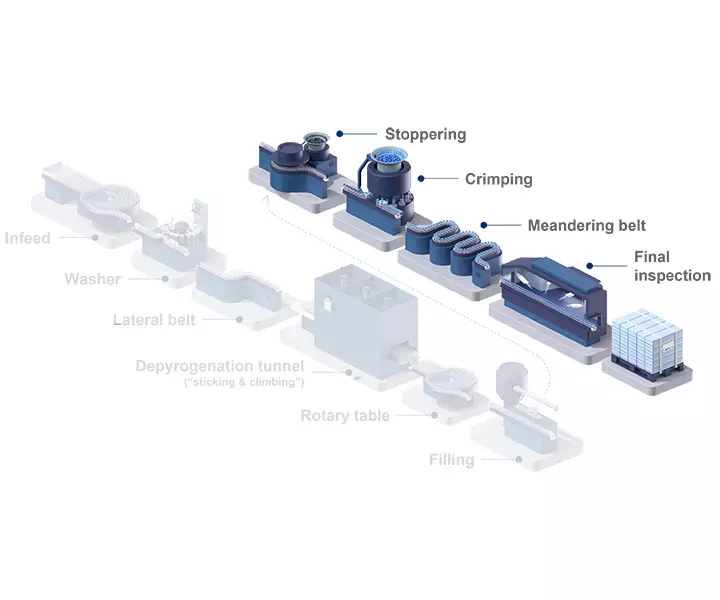
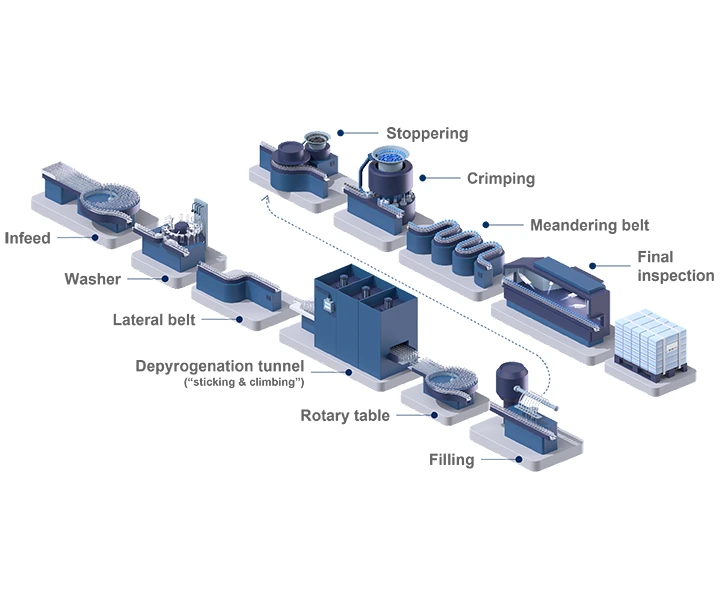
Today’s bulk-filled vials pose multiple cost challenges to pharma companies. These costs include cleanroom space, washing and WFI usage, as well as sterilization and energy. By simplifying supply chain operations and outsourcing value chain steps, pharma companies can simplify their manufacturing processes, streamline their production footprint, and focus on their core business.
> Discover how sterile glass vials can reduce the Total Cost of Ownership
Detect and eliminate your areas of risk in your filling process to improve patient safety
Between 2017 and 2021, particles and insufficient sterility assurance were responsible for 50% of the 396 product recalls in the market related to parenteral drugs (Source: FDA). Thus, making them the most common causes for such recalls. Today’s bulk filling lines can create cosmetic defects on the turning plates or during transportation, but since RTU vials are nested in a tub, they can be processed without glass-to-glass contact, resulting in zero deterioration in cosmetic quality. This also significantly reduces the generation of particles and cuts the risk of breakages on the filling line.
> Find out more about how to reduce particle reduction in RTU packaging
However, caution is required when choosing RTU containers, as the secondary packaging (tub, nest, header bags, and sealing or lid material) is an additional potential source of contamination. Therefore, a holistic approach to contamination control is required. Learn how SCHOTT Pharma improves patient safety with the right sterile secondary packaging materials:

How the nest material can reduce particle contamination
Nest designs of RTU drug containment solutions vary depending on the container type, but they all have a common purpose: To keep the containers in place during storage, transportation, and handling, as well as to avoid glass-to-glass contact, and present the container precisely to the filling needle. A systematic approach for selecting the best nest material is vital to improving the quality of RTU vials significantly.
DOWNLOAD WHITEPAPERHow the right lid material and adhesive can reduce particle contamination
The application of "peelable" adhesives to the roll stock and die-cutting may result in loose fibers and adhesive artifacts, as the tub sealing lid is in immediate proximity to the nested containers and closures. Selecting the right combination of lid material and adhesive, along with strict process controls, influences the performance of the entire RTU packaging system.
DOWNLOAD WHITEPAPERHow the nest material can reduce particle contamination
How the right lid material and adhesive can reduce particle contamination
Maximize your patent lifetime with sterile drug containment solutions
Switching from bulk to RTU containers can significantly maximize patent lifetime and cash flow by entering the market earlier. As new drugs are commercialized, production is not only scaled up but also scaled out, with manufacturing taking place in parallel across a number of facilities to supply local markets.
Key to this strategy is a standardized machine concept that can operate with pre-tested and standardized ready-to-use (RTU) packaging components. The use of RTU packaging eliminates the need for frequent changeovers between batches, which can seriously reduce the overall equipment effectiveness (OEE) of traditional filling lines.
> Learn more about how RTU packaging accelerates time-to-market
Rethink your fill-and-finish processes now
The adaptiQ® RTU vial platform from SCHOTT Pharma enables flexibility while simplifying and increasing the speed of processes, thus saving costs. Implementation, validation, and the ramping up of new filling lines can be carried out in shorter time scales, while enhancing the quality of the containers. When choosing the primary packaging and the corresponding fill-and-finish concept, medical safety and economic viability must be considered. The benefits of careful decision-making at an early stage cannot be overstated.Let’s rethink your processes together!