Choosing the right drug containment solution
From development to commercialization: drug containment solutions for every stage
Bringing life-saving medications to the market is a complex journey with multiple challenges. Choosing the right primary packaging solution is one of these challenges, and something that’s crucial in the early drug development stages. With limited in-house capabilities primarily focused on drug research and development, biotechs and other innovators such as universities and small pharma companies typically need support with container selection and the early evaluation of drug-container interaction.
But it’s not just small companies that need help. Large, established pharma companies also require a simple way to access a variety of primary packaging options at an early stage to find the right solution for their drug. With the rise of personalized medicines and tighter requirements for compounding pharmacies, the industry is moving away from large-scale production and towards small batches, where flexibility, ease of use, and availability are essential.
SCHOTT Pharma has responded to this trend by offering a wide range of containment solutions that are pre-sterilized and available in small quantities, supporting companies at all stages of their drug development journey.
How standardized processes enable pharma companies to concentrate on development
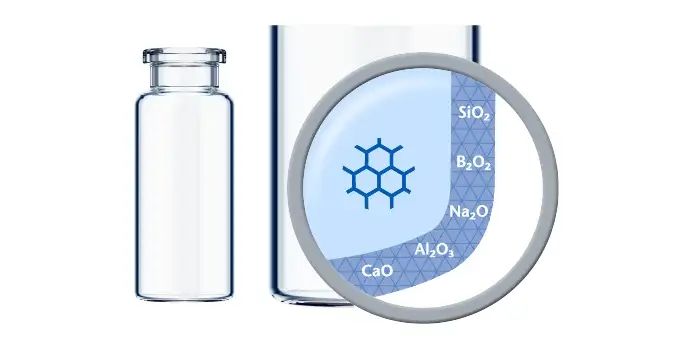
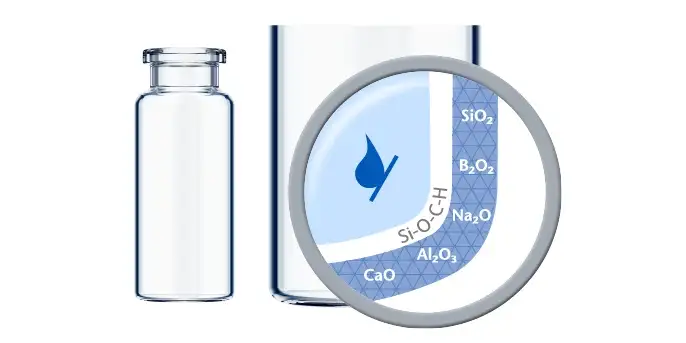
Advances in science are revolutionizing the medicinal landscape, offering unparalleled opportunities to combat and mitigate disease. As drug developers dive into this realm of progress, they encounter new analytical and technological hurdles. Many of these formulations are highly sensitive so increased attention needs to be paid to their primary packaging thanks to the potential impact of drug-container interaction.
The seven top challenges of drug-container interaction for biologics and biosimilars
With greater demands on the container for increased patient safety and convenience, drug developers strive to mitigate risks while gaining drug development and lifecycle flexibility. Using standardized processes that simplify the drug lifecycle, biotechs and other innovators can focus on improving patients’ health.
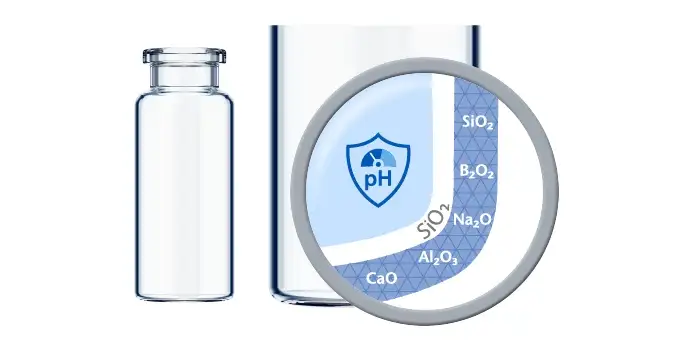
Mitigate and eliminate areas of risk with pre-tested products
Patient safety is intrinsically linked to controlling particles and sterility during fill-and-finish, with most recalls of parenteral drugs issued by the FDA over the course of four years (2017-2021) due to these factors. This shows they are paramount when producing parenteral drugs.
Today’s bulk filling lines can create cosmetic defects during transportation or from turning plates. Drug manufacturing using RTU drug containment solutions such as vials eliminates vital risk areas for patient safety by outsourcing to standardized processes. RTU drug containment solutions are nested in a tub to be processed without any glass-to-glass contact. This results in zero deterioration of cosmetic quality and a reduction in particle generation, as well as a decrease in the risk of breakage.
However, caution is required when choosing an RTU drug containment solution as the secondary packaging is an additional potential source of foreign contamination. Therefore, a holistic approach to contamination control is required.
Five steps for particle reduction in RTU drug containment solutions
Maximize patient benefit by accelerating time to market
The pharmaceutical manufacturing industry has undergone a significant transformation in recent years. The rise of biologics, biosimilars, and targeted therapies for rare diseases with smaller patient populations has reshaped the market. This shift has resulted in the adoption of smaller batch sizes and an increased demand for frequent changeovers on fill-and-finish lines.
Standardized tubs accompanied by pre-tested, pre-washed, and pre-sterilized RTU drug containment solutions offer a fast time to market and an efficient ramp-up of filling capabilities during commercialization to meet these evolving needs. Throughout the entire drug development process, from research to clinical trials and commercialization, RTU drug containment solutions offer:
- No requirement to build your own washing/sterilization process and footprint.
- Swift changeovers between different container types and formats without switching the filling line.
- Quick technology transfers to various filling lines, sites, and contract development and manufacturing organizations (CDMOs) thanks to a high level of standardization.
Find out more in our blog article “How RTU packaging speeds up time to market”
RTU vials have gained popularity in clinical and small to medium commercial settings. Big pharma companies and CDMOs widely adopt RTU drug containment solutions for large commercial settings to advance aseptic manufacturing practices, with higher drug yields from fewer resources.
adaptiQ® RTU vials can be ordered directly from the SCHOTT Pharma Online Shop, which offers rapid delivery for the smallest quantities needed for your clinical trial. With our adaptiQ® Fast Track Kits, you receive a comprehensive solution for small-batch filling. Each kit includes RTU vials, pre-tested stoppers, and caps, guaranteeing the safe storage of your drug products.
Get in touch
If you have any questions or require further information, we are happy to help.