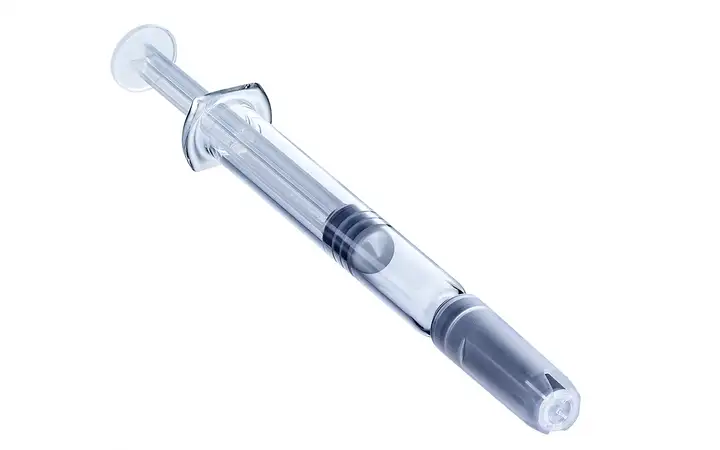
SCHOTT Pharma Syringes Ready-to-use prefillable polymer and glass syringes
Contact usPrefillable syringes for safe, precise, and reliable drug storage and injection
Whether made of glass or advanced polymer, SCHOTT Pharma’s prefillable syringes (PFS) offer a highly stable, long-term storage solution and a safe, convenient delivery system for patients and healthcare professionals.
By reducing the number of manual preparation steps compared to conventional packaging, PFS significantly lower the risk of medical errors and contamination, enhancing safety and reducing drug waste.
SCHOTT Pharma’s syringe portfolio is built on a flexible approach to materials, offering two complementary platforms:
- syriQ® and syriQ BioPure® – made of gold-standard FIOLAX® Type I Borosilicate Glass, characterized by strong barrier properties, reliable functionality, a proven regulatory pathway, and high compatibility with a range of fill-and-finish systems.
- SCHOTT TOPPAC® – made from advanced Cyclic Olefin Copolymer (COC), characterized by its lightweight design, superior break-resistance, excellent barrier-properties, and design freedom that allows customized packaging design and ensures flawless device integration.
This dual offering enables pharmaceutical companies to select the most appropriate solution based on drug formulation, delivery method, and application setting, ensuring optimal performance without compromise.
Both platforms are supported by SCHOTT Pharma’s global expertise, regulatory support, and system-level integration, making them ideal for a wide range of therapeutic applications.
Find the product that fits your needs:

Ready-to-use prefillable glass syringes for vaccines
syriQ® glass syringes combine proven container closure integrity, excellent gliding performance, and reliable functionality, offering a dependable solution for storage (down to -50 °C) and the administration of a wide range of vaccines. SCHOTT Pharma syringes offer an extensive range of options, including the proven syriQ® Rigid Cap (SRC) closure system, allowing syriQ® to be perfectly tailored to individual vaccine requirements.

Ready-to-use prefillable glass syringes for biologics
syriQ BioPure® glass syringes are designed for the safe and reliable administration of highly sensitive biologics. Low silicone levels combined with tight dimensional tolerances ensure consistent gliding forces and injection times, making them ideal for autoinjector and homecare use.

Ready-to-use prefillable and silicone-free glass syringes
syriQ BioPure® silicone-free glass syringes are designed for silicone-sensitive biologics, with both syringe barrel and plunger free of silicone. Enhanced by the absence of silicone oil, the proven quality level of syriQ BioPure® opens up new possibilities for the storage and administration of silicone-sensitive drugs while improving patient safety.

The polymer syringe platform
Made from break-resistant COC material, SCHOTT TOPPAC® syringes combine versatile drug delivery solutions with decades of pharmaceutical expertise, ensuring reliability and performance for critical medical care.

Ready-to-use prefillable polymer syringes for infusion therapy
Compatible with most common syringe pumps, this PFS sets new standards in overcoming the challenges in hospital environments.

Ready-to-use prefillable polymer syringes for aesthetic injections
These RTU prefillable polymer syringes for aesthetic treatments are engineered to deliver consistently low break-loose and gliding forces, ensuring accurate dosage and reliable performance.

Ready-to-use prefillable polymer syringes for deep-cold storage
A polymer-based prefillable syringe solution engineered for deep-cold drug applications, which maintains full functional integrity and performance under cryogenic conditions.

Ready-to-use prefillable polymer containers for medical applications
SCHOTT TOPPAC® unique offers a range of advanced customization options, enabling you to design the ideal container for your needs.
Pharmaceutical prefillable syringe solutions for modern drug delivery
Supplied pre-sterilized in an industry-standard nest and tub configuration, SCHOTT Pharma syringes support simple, high-speed filling operations. Designed to meet the increasing requirements on primary containers for pharmaceuticals, such as sensitive biologics or the application shift from hospital to home care, our syringes ensure the safe handling of highly sensitive medications and offer wide compatibility with injection devices.