Contamination control for aseptic filling
Pioneering aseptic filling with ready-to-use packaging for enhanced contamination control
As regulatory requirements become increasingly strict for the pharmaceutical industry, contamination control and the need for improved aseptic filling solutions become more important. This applies to all systems, not just those with high automation. In line with optimized fill-and-finish processes, improvements can be made in ready-to-use (RTU) packaging. SCHOTT Pharma overcomes the limitations of current RTU options by using superior materials and a design that ensures sterility protection and seal integrity.
Next-level sterile integrity for aseptic filling
Flexible fill-and-finish processes often require sterile packaging. However, standard solutions increase the risk of contamination during transport and transfer into the aseptic environment. In line with optimized fill-and-finish processes, improvements are being made in RTU packaging.
SCHOTT Pharma follows a quality-by-design approach to overcome the limitations of current RTU options, using superior materials and a design that ensures sterility protection and seal integrity. Our RTU containers provide next-level sterile integrity, enhance safety measures, and reduce the risk of contamination to meet increasingly strict regulatory requirements.

The difficulties of contamination control
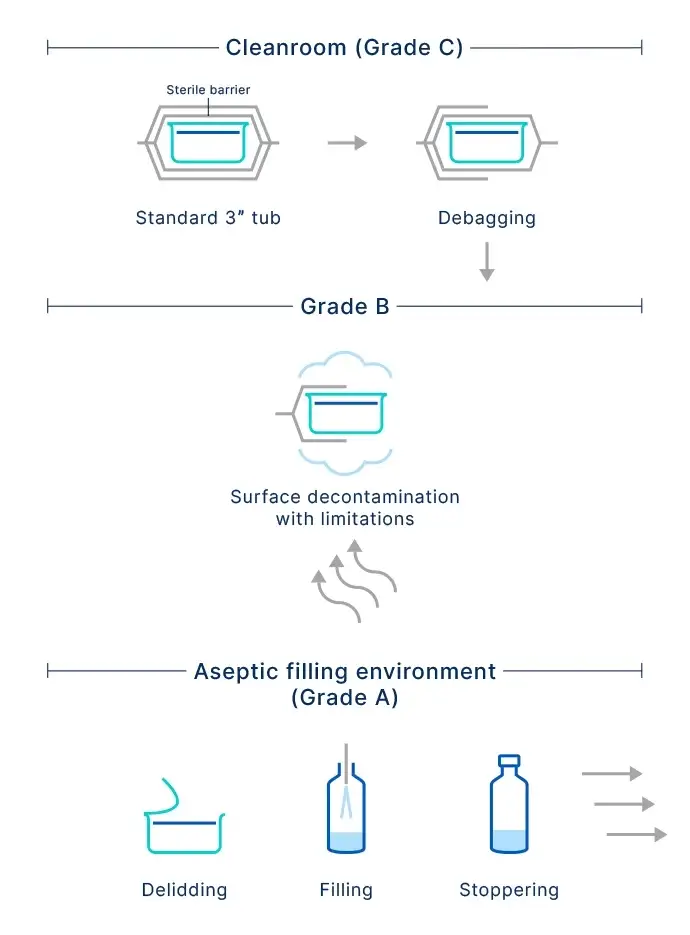
Handling and transporting RTU containers to ISO 5 (Grade A) cleanroom zones poses a contamination risk and can be difficult to manage. To reduce the risk of contamination and human error, interest is growing in the continuous improvement of fill-and-finish processes, and the preceding transfer steps into the aseptic zone.
As current RTU packaging solutions do not prevent contamination before the aseptic filling zone, pharma companies have to rely on corrective actions, such as surface decontamination.
But each decontamination option has its disadvantages. E-beam sterilization comes with high capital and operating expenses, generates ozone, and requires staff with radiation safety management expertise. Hydrogen peroxide is a batch process, which requires a high capital outlay and may leave harmful residuals. Ultraviolet irradiation is sensitive to shadowing, has low compatibility with tub handling, and requires frequent replacement of the light source. Finally, the manual process using alcohol wipes is difficult to validate and leaves residuals.
Contamination control using SCHOTT iQ® Integribag
SCHOTT Pharma has overcome the limitations of current RTU options with the development of the SCHOTT iQ® Integribag, which uses superior materials and a design that ensures sterility protection and seal integrity. Based on a quality-by-design approach to enhance sterile integrity, the robust double-bag system supports NTT and eliminates the need for a decontamination step, as well as unnecessary time and resources.
The design features two separate bags, each consisting of a Tyvek® component in the front and a blue-colored laminate on the back. The intricate combination of layers of industry-leading materials and outstanding production processes provides an improved physical barrier and enhanced contamination control. This innovative new sealing technique not only enhances sterility, but improves the inspection of seal integrity using visual methods.
More importantly, the qualified double-bag design ensures that the sterile barrier encompasses the outside of the tub, as well as the Tyvek® seal layer.

Frequently asked questions:
E-beam sterilization or electron beam sterilization as a safe and effective method used to sterilize a wide range of different medical devices and pharmaceutical containers. it is considered one of the most cost-effective methods for low density products.