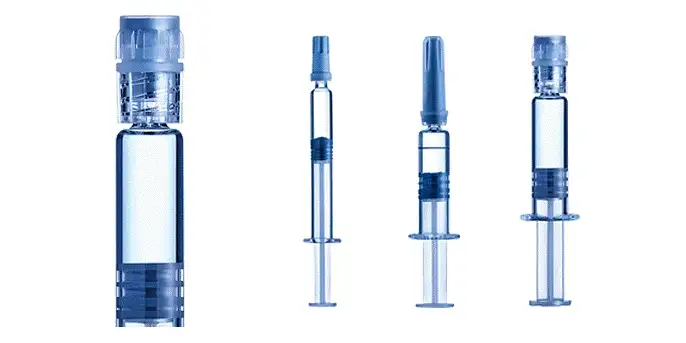
mRNA Storage - Finding the Ideal Solution
Selecting vials for speed to market or prefillable syringes (PFS) for ease of use and flexibility. Industry-standard syriQ® glass PFS are proven at temperatures down to -50°C and SCHOTT TOPPAC® polymer PFS down to -180°C, making them a great solution for the storage of mRNA drugs. We have the solution to support your mRNA storage and transport needs.
mRNA applications are a challenge for primary packaging
The demanding low temperature storage and transport conditions of mRNA applications put additional strain on the primary packaging system container. Cold chain conditions and the freeze-thaw cycle can impact mechanical stability, performance, container closure integrity and particulate levels. All these potential risks must be understood to launch an mRNA drug with confidence.
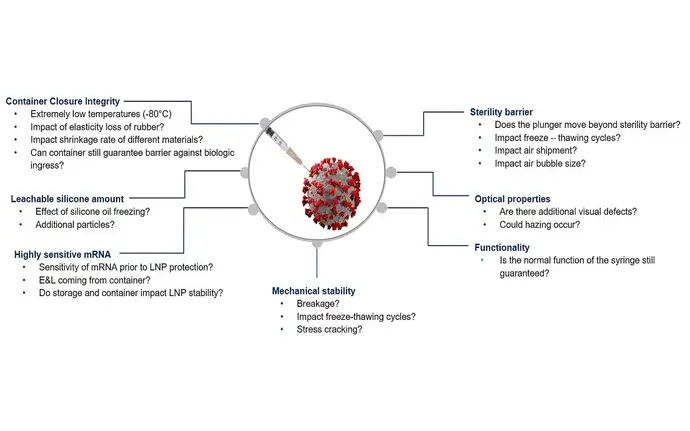
Overcoming mRNA application challenges with SCHOTT Pharma
Fast time-to-market? Using a PFS for more convenience? Extremely cold and challenging supply and storage conditions? Issues with drug container interactions? Technical and unbiased support?
Data and results
Testing data for various topics like sterility, CCI, potential drug-container interaction or functionality helps to better understand the aspects of a safe mRNA storage.
Polymer syringes ideally suited for low temperature mRNA applications.
Sterility barrier can be controlled with the right syringe components and F&F parameters.
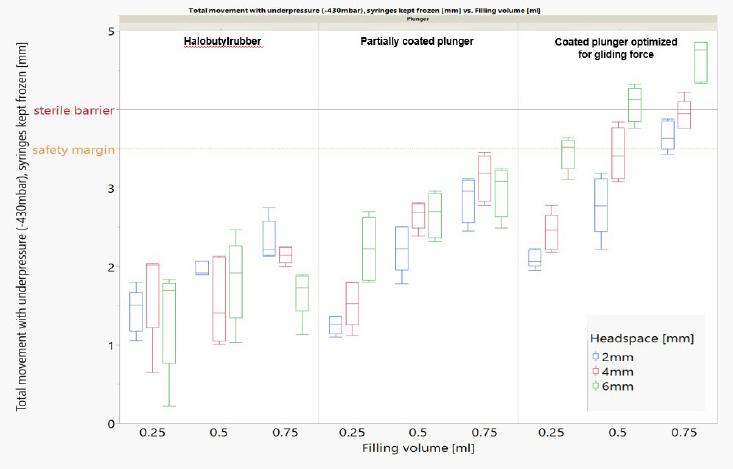
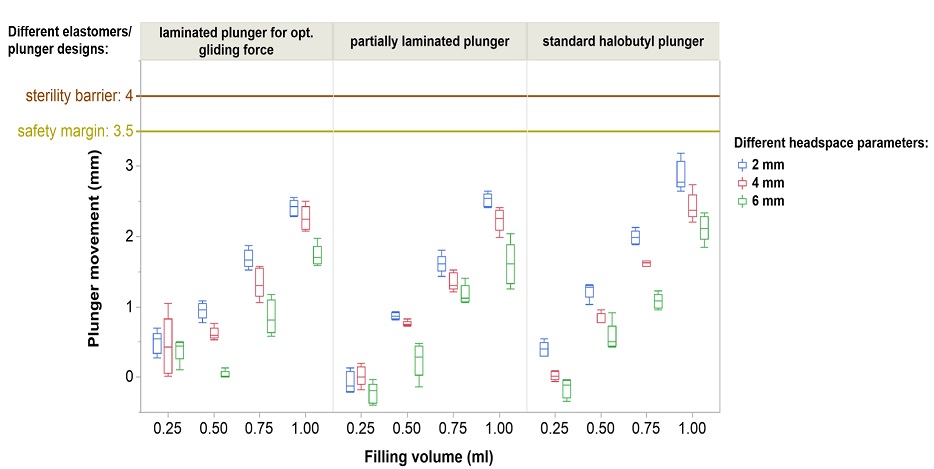
*Headspace size, the type of plunger as well as the filling volume impact the plunger movement.
Container closure integrity (CCI) is maintained even at -180°C.
Reduced risk for drug interaction and sub-visible particles with a cross-linked siliconization process.
| Time | Free silicone [mg/L] 3x frozen at -20°C and thawed |
Free silicone compared to standard SCHOTT TOPPAC® |
Free silicone [mg/L] stored at 5°C |
Free silicone compared to standard SCHOTT TOPPAC® |
|||
|---|---|---|---|---|---|---|---|
| SCHOTT TOPPAC® cross-linked siliconization Standard cross-linked silicone |
0d | 0.23 | N/A | < 0.2 | N/A | ||
|
SCHOTT TOPPAC® sprayed siliconization Sprayed on DC360, 0.55 mg/barrel |
0d | 5.6 | 24 times | 1.09 | 5 times | ||
*After 3 cycles of freezing and thawing at -20°C, the leachable silicone quantities increase for both siliconization technologies, but sprayed silicone is much more affected. We see 24 times more free silicone oil for the syringe siliconized with sprayed on siliconization. The lubrication technology matters for drug stability. An immobilized cross-linked siliconization lowers the risk for any drug interaction.
Normal syringe functionality even in deep-cold temperatures.
All normal polymer syringe functionalities remain unchanged after 3 times freeze/thaw and no residual risks were identified after several studies:
- No change of transparency
- No decrease in mechanical stability
- No change of optical appearance
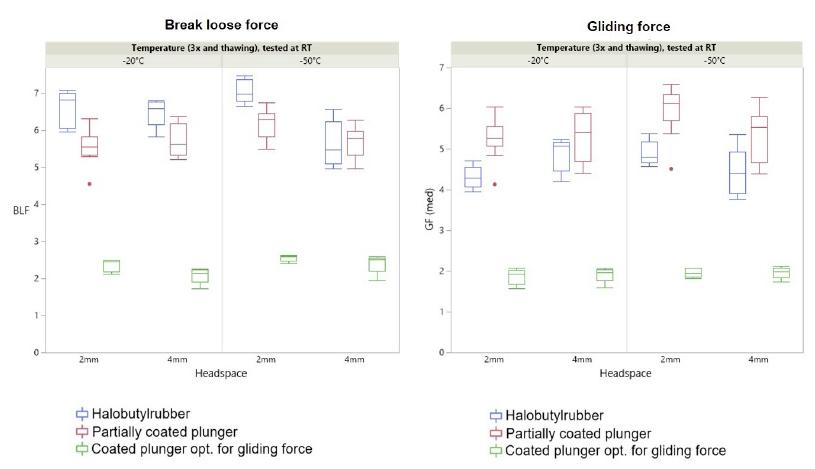
*No difference in break loose and gliding force was observed at the different temperatures and results were comparable to syringes stored at room temperature. Indicating a freezing-thawing cycle doesn't have a significant impact on functionality.
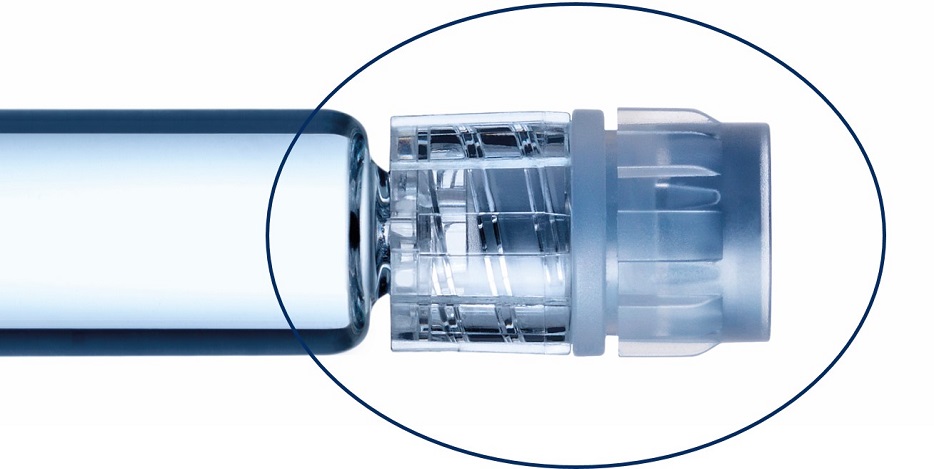
Glass prefillable syringes ideally suited for low temperature mRNA applications down to -50°C.
None of the tested plungers shown here breach the safety margin: Deep cold temperatures have a complex effect on plunger movement. We proved the integrity of the sterility barrier with different controlled fill-finish parameters.*
*syriQ® 1ml long glass syringe with SRC; WFI fill medium shown here. Data for other systems available.
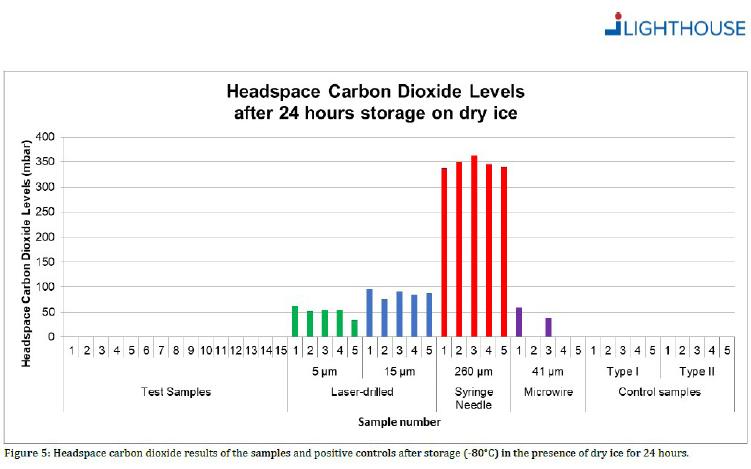
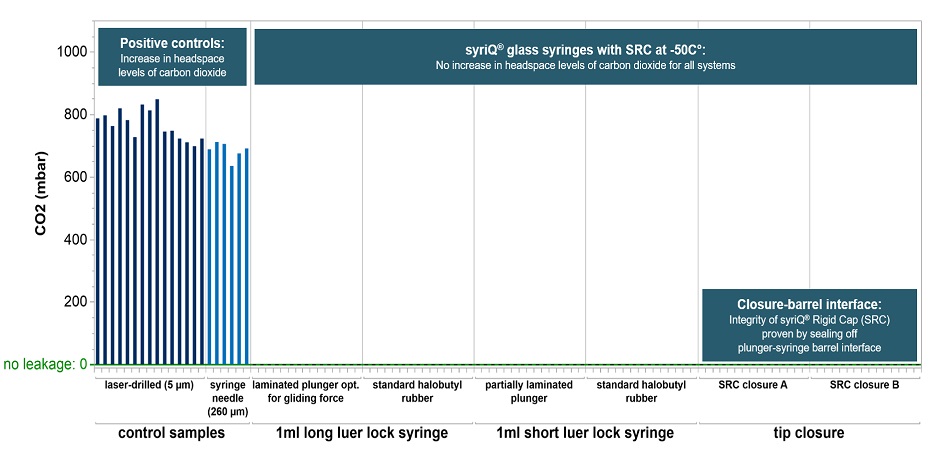
A widely-referenced test method* based on headspace analysis proved container closure integrity down to -50°C for different glass prefillable syringe systems (PFS).
* For each PFS system, 15 empty syringes were stored for 24 hours on dry ice at -50°C. The syringes were tested with qualified Lighthouse instruments FMS-Carbon Dioxide Headspace Analyzer (Model FMS-CO2). No test samples showed signs of CO2 ingress.
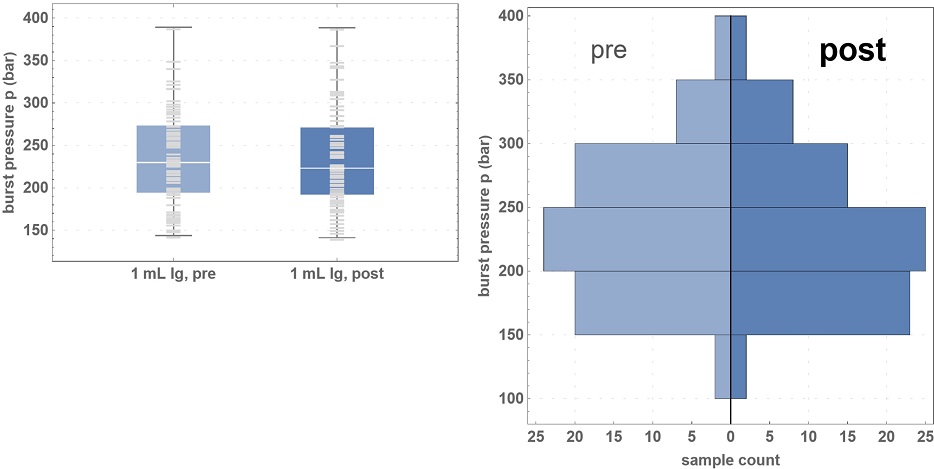
Burst pressure results showed mechanical strength resilience through the freeze-thaw cycle.*
*75 syringes tested pre- and post-freezing at -50°C, using buffers and a freeze-thaw cycle designed to simulate the mRNA cold chain.
Torque strength and Luer Lock adapter connectivity were unaffected, and out of 900 syringes we observed no changes to optical properties.
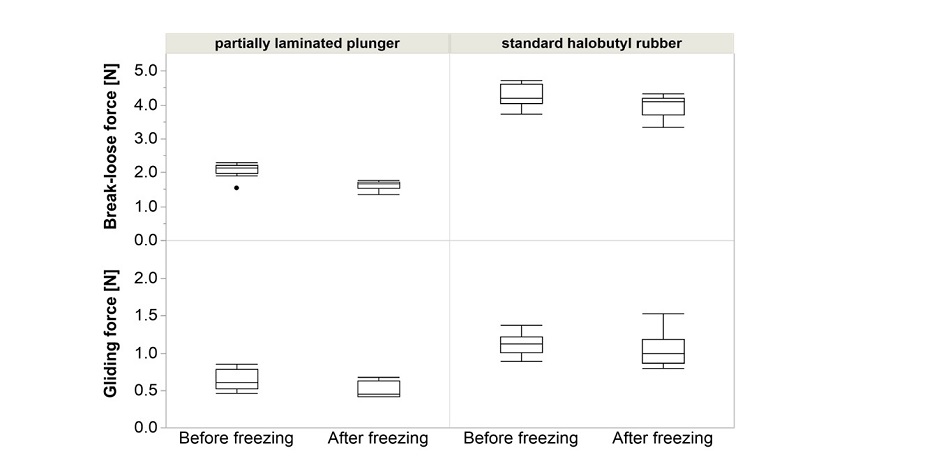
Injection performance (break-loose & gliding force, BLGF) for syriQ® glass syringes is minimally impacted following the freeze-thaw cycle in the deep cold temperature range.
*Example: syriQ® 1ml long glass syringe with SRC; data for other systems available.
SCHOTT Pharma as a right partner for mRNA technology
- Optimal drug containment solutions available for every life cycle phase: from vials to prefillable syringes.
- Unbiased consulting and support for pre-fillable syringes depending on the application's need.
- SCHOTT Pharma Services provides drug container compatibility testing for multiple drug developments
Frequently asked questions
As mRNA drugs are becoming increasingly popular in the pharmaceutical industry, they are now used to treat genetic diseases, viral infections, and COVID vaccines. Their potential is yet to be fully taken advantage of, especially when it comes to the use of mRNA drugs in vaccines.