Drug submission preparation
CONTACT USNavigating the complexities of pharmaceutical drug submission preparation
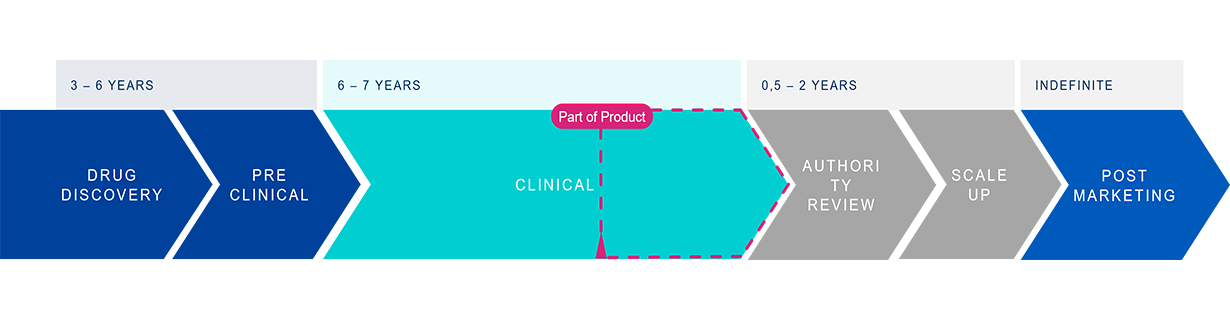
The preparation for regulatory approval of new drug products demands thorough packaging-related testing to ensure drug safety and efficacy. This testing spans a wide range of evaluations, including material characterization, drug-container interaction, container closure integrity, and functional performance. Such comprehensive testing is essential not only for meeting stringent regulatory standards, but also for safeguarding patient safety.
Streamlining testing processes for regulatory compliance and accelerated submission
Pharmaceutical companies often face challenges in their drug submission process, balancing internal teams and external labs for their testing needs. While internal teams are deeply involved in development, they may lack expertise in some specific testing procedures. On the other hand, external labs offer specialized knowledge for testing methods but often pose coordination and result integration challenges, leading to a fragmented process with inconsistent results.
Dealing with internal resource shortages and managing external suppliers becomes a risk to fail fast time to market. With the complexity and the high stakes involved in the submission preparation phase, pharmaceutical companies shoulder a significant responsibility, ensuring that highest degree of patient safety is achieved under the tightest time constraints.
Four tests for your drug submission preparation
Material compatibility testing is fundamental to ensuring that the material candidates are qualified to be used in the drug containment application. Material compatibility testing serves to:
- Adhere to regulatory requirements – ensuring compliance with global standards for material use in pharmaceutical packaging.
- Match intended specification – confirms parameters such as chemical composition and layer thickness (e.g. silicone or other coatings).
This involves rigorous assessment of the material characteristics of the glass, polymer, or elastomeric primary packaging components. Testing procedures include:
- Compendial testing – standardized tests to ensure material compliance with requirements defined in the pharmacopoeias.
- Packaging component characterization – tests include the measurement of layer thickness or evaluation of the total or easily removable amount of silicone.
- Particle analysis – characterizes cleanliness of materials and reveals sources of potential contamination from manufacturing.
Drug interaction with the containment solution is a critical risk to the drug’s efficacy. Proper evaluation verifies the compatibility of the drug formula with the system’s contact materials. Drug interaction testing serves to:
- Ensure drug efficacy – verifying that the container does not alter the therapeutic effects of the drug.
- Ensure safe storage – confirming the absence of critical toxic by-products from drug-container interaction during storage.
Drug-container interaction testing involves detailed analysis of how the drug interacts with the container, predicting potential chemical and physical changes during storage time. Testing methods include:
- Delamination studies – assesses the risk of material degradation and its impact on the drug.
- Particle analysis – counts and characterizes particles resulting from drug-container interaction.
- Extractables studies – evaluates potential chemicals that might leach from the container.
- Leachables studies – investigates the impact of leachables on drug stability and patient safety.
- Elemental impurities analysis – ensures no harmful elements are present in the packaging.
Effective container closure integrity is critical to safeguarding the sterility and quality of a pharmaceutical product across its lifetime. Proper testing enables the assessment of a drug product’s packaging for the risk for leakages. CCIT serves to:
- Ensuring drug efficacy and safety – it verifies the system design to support the sterility and stability of a pharmaceutical product.
- Proof regulatory compliance – it aligns with global standards, including FDA and EMA guidelines.
- Confirms robust design – it proves effectiveness against contamination and integrity breaches.
CCIT ensures that containers such as vials and syringes are impermeable to contaminants, which is a vital aspect of patient safety and drug efficacy. CCIT includes the following tests:
- Dye penetration test – identifies microscopic leaks in container seals to verify seal integrity.
- Headspace analysis – assesses the change in gas composition within sealed containers over time to determine the ability of gas to permeate into the container closure system.
Functional testing validates a product’s design to satisfy the application-specific user requirements and regulatory standards. Functional testing serves to:
- Assure performance – it validates the container’s operational reliability and effectiveness.
- Ensure system compliance – it matches stringent industry and safety regulations.
This group of tests validates the system’s design in providing reliable functionality under the specific conditions of the intended use and across the entire product lifetime. This is especially relevant for combination products such as drugs in prefilled syringes or injection devices. Typical test methods characterize the system for:
- Break loose and gliding force – assesses the ease of dispensing.
- Deliverable and residual volume – for accurate dosage and minimal waste.
- Liquid leakage – ensures container integrity under a variety of conditions.
- Septum resealability – validates the closure’s ability to reseal post-penetration.
- Flange and Luer Cone breakage resistance – tests the mechanical strength against breakage.
SCHOTT Pharma’s PartnerLab: Pharmaceutical testing for regulatory submission
Understanding the importance and complexity of the pharmaceutical industry’s regulatory submission process is key. Staffed by experienced professionals, our state-of-the-art, FDA-registered laboratory simplifies and streamlines this process. With PartnerLab, we offer a comprehensive suite of testing services designed to meet the diverse and stringent requirements of drug product packaging and interaction testing.Beyond testing, we team up with you to ensure the safety and success of your product. By entrusting us with the complexities of testing and compliance, you can focus on your core strengths, confident that your performance testing is expertly handled. PartnerLab streamlines your testing process, accelerates your market submission, and elevates the assurance of your product’s quality and safety.