
Biologics and biosimilars Understand how to bring biologics to market, from research to regulatory compliance.
How to bring biologics and biosimilars to market
The increasing prevalence of chronic diseases and the aging population have created a growing demand for effective and innovative treatments. For chronic diseases such as autoimmune disorders or cancer, and those associated with old age, such as rheumatoid arthritis, biological drugs offer effective long-term solutions for patients, improving their quality of life.
As our understanding of the mechanisms of diseases improves, researchers can identify specific paths and molecular targets for intervention. Due to their suitability for targeted disease management, biological drugs are often preferred over traditional medications, as they frequently exhibit higher effectiveness and fewer side effects. Advances in biotechnology, including genetic engineering and recombinant DNA technology, have expanded the range of diseases that biologics can treat.
The individualization of biological drugs tailored to individual patient profiles allows for personalized treatment plans, particularly relevant in oncology and other areas where genetic variability plays a significant role. The continuous development of new biological drugs broadens the treatment options available to patients and physicians.
Regulatory agencies have established frameworks for the development and commercialization of both original ‘innovator’ biological drugs, and Biosimilars (an officially approved version of an ‘innovator’ product which is manufactured after expiry of the original product patent).
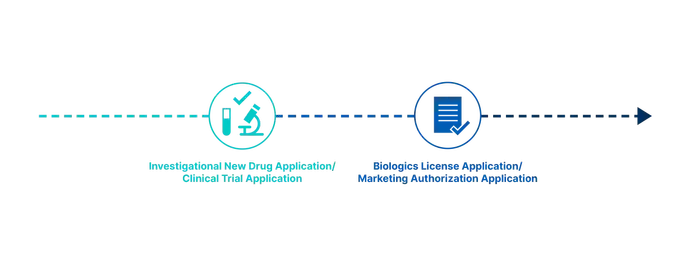
Nevertheless, for drug development professionals, there are two major regulatory hurdles on the road to commercial success: the clinical trial application, which defines the scope of the clinical testing phase; and the marketing authorization application, which proves compliance. (In the USA, these are known as the investigational new drug Application and the new drug application respectively.) Approval by the regulatory authorities for both is obligatory. The challenge is that the regulations and standards for entry differ for each regional or international market.
Containment and delivery for biologics
Despite their advantages for treating disease, biological drugs also bring new challenges for primary packaging. An experienced guide will help you reduce the time to market and maximise the value of patent protection.
Sensitivity
Contamination
Storage temperature
Container interaction
Compatibility
Dosage
Administration
Partnership based on knowledge and experience
We promote human health by collaborating with drug developers to create complete containment and delivery solutions for biologic and biosimilar drugs. Thanks to our detailed knowledge of materials, markets and regulations we can show you an effective path to achieving compliance for your primary packaging. We are experts in every stage of development for drug containment and delivery solutions: from design & selection; through clinical testing; to commercialisation and production.
Pre-Clinical Research
The objective of the pre-clinical phase is not simply to identify viable formulations. Based on their research, the R&D team also submits a clinical trial application which accurately describes the drug and the scope of testing on humans. In this context, the drug must work in tandem with primary packaging to provide safe & effective container for storage, delivery and administration, too.
Container design
We can turn your concept into a reliable containment solution, by evaluating the feasibility of ideas, testing prototypes & documenting development with Design History Files.
Learn more about drug containment and delivery services
Container selection
Analytical services from our independent laboratories will validate the compatibility of your containment solution for the requirements of your specific drug product.
Learn more about analytical testing services
Clinical phases I, II, III
These test phases ensure that only safe and effective medications reach the market. A series of carefully designed trials on human subjects, determines whether the drug, its primary packaging and its administration mechanisms treats the targeted medical condition safely and effectively; and identifies risks or adverse reactions.
Clinical testing
Analytical testing validates the compatibility of your containment solution with the requirements of your specific drug product. This generates objective data to support the selection of the ideal containment solution, solve root-cause challenges, and prepare regulatory-compliant evidence about containers and combination devices for new drugs.
Learn more about analytical testing services
Independent laboratories
Rigorous testing at our accredited facilities examines key aspects such as the compatibility between drug and materials, packaging performance, or container closure integrity testing (CCIT) and includes document management prior to submission.
Learn more about SCHOTT Pharma's PartnerLab
Post-launch
Turning a promising drug candidate into a viable product that generates revenue and profit requires more than just successful compliance. You will need to plan the scale-up and scale-out of production facilities carefully to ensure effective manufacture and distribution.
From early samples to commercial production
For customers without the capability to fill samples for testing, we offer flexible fill-and-finish operations for syringes, cartridges and vials. Scalable production enables you to match the growing volumes required for early-stage testing, or clinical testing through phases I, II and III.
Learn more about fill-and-finish services
Scale-up and scale-out
After approval, we can quickly scale-up as production transitions to full commercialisation while maintaining the highest standards of container quality, safety, and efficacy. Our manufacturing and support network permits scale-out of primary packaging production across 16 global locations, to avoid disruptions and ensure stable and consistent supply. Once a new drug is brought to market, production filling in RTU format can also be scaled out effectively. Sourcing via multiple CMOs - with fill-and-finish taking place in parallel across a number of facilities to supply local markets – is fast path to scale-out, and reduces the risk of international supply-chain interruptions.
Learn more about RTU and time-to-market
Containment solution choices
When you want to explore the choices that are open to you, SCHOTT Pharma is ready to assist. We have hands-on experience of a wide range of drug containment and delivery materials, formats, shapes and sizes.
SCHOTT FIOLAX® is a Type I Borosilicate that continues to be the de facto quality standard for glass in the pharmaceutical industry. And our advanced Cyclic Olefin Copolymer (COC) solutions lead the way in break-resistant, lightweight, transparent syringes that come with full regulatory support and decades of expertise.








