Validate sterility in RTU packaging
Validating sterility in RTU packaging is key to understanding the design of sterilization process and their impact. It is important to realize that sterility is about probabilities rather than absolute values. This means how great is the probability that a sterilized object will have surviving organisms on it, and what are the consequences to the "product" because of this guarantee, e.g., dangerous levels of sterilant residuals and/or detrimantal effects on the packaging materials?
The definition of sterility
Sterility is quantified by the Sterility Assurance Level (SAL), which indicates that a product has been rendered sterile. An SAL of 10-6, for example, is the level required by the US FDA for medical devices and injections under the definition of an “overkill” approach. According to USP, “overkill” sterilization can be defined as a method in which the destruction of a high concentration of a resistant microorganism supports the destruction of reasonably anticipated bioburden present in routine processing.” It is incumbent that the manufacturer of “sterile” components monitor and trend bioburden.
The SAL is determined by challenging the sterilization cycle with high levels of resistant organisms and quantifying the number of any surviving colony forming units (CFUs) after sterilization. From an uncontrolled process, there is typically a large variation in the nature and number of CFUs on the product as presented to the sterilization cycle. The variation is determined by monitoring the product(s) pre-sterilization for levels of bioburden and speciation of the isolates (organism ID) to determine the inherent resistance to the sterilization modality. Married surfaces, where two materials fit tightly together (like the needle shield and the syringe hub), and small lumens (narrow passages such as the inside of a small-bore needle), represent significant challenges to “demonstrate” the sterilant will successfully kill bioburden in these hard-to-reach locations.
Validate sterility in the RTU packaging: the consequences of high bioburden
If monitoring and trending programs determine that “the product” contains high levels of bioburden, high levels of variation in bioburden, or high levels of resistance, regulations require manufacturers to address upstream processes, e.g. cleaning programs, cleaning frequency, microbiological cleanliness of the packaging materials, and assembly processes and the selection and rotation of antimicrobial agents. If bioburden is not under control, the sterilization cycle may need to be adjusted to mitigate the additional risk. As it is subjected to sterilizing conditions, the inherent bioburden will gradually be killed off and the number of surviving organisms reduced and hopefully eliminated. After a certain amount of time and under the right conditions (temperature, pressure, humidity, time, and gas concentration), the probability of survivors will reach 10-6 spore reduction, which signifies that there is an incredibly low chance that a single viable organism survives the sterilization cycle.

External Process Challenge Devices (EPCDs) containing certified Biological Indicators (BI) containing >1x106 highly resistant bacterial endospores (verified for each lot by population verification testing) are placed on every pallet in every cycle. After the cycle, the biological indicators are removed and transferred to a growth-promoting medium. After incubation, if no growth is present, you can be confident that the sterilization cycle has reached the required SAL. This, along with parametric review of the cycle processing record, provides us with the assurance that the sterilization cycle was successfully executed according to validated parameters.
Note: Biological indicators are available at different SAL concentrations, prepared from different organisms, with different inherent resistance, to challenge different sterilization modalities (heat, steam, EtO, E-beam, VHP, H2O2, and Gamma).
A sterile component will maintain sterility along the value chain through the use of validated processes and appropriately designed primary and secondary packaging.
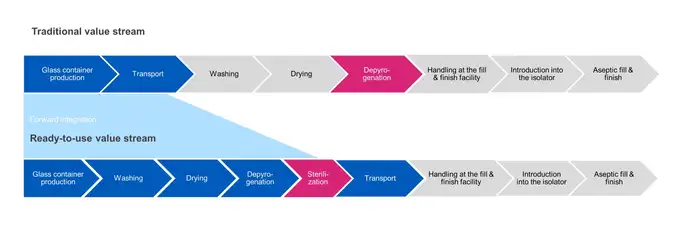
In traditional bulk filling, sterilization of the container is carried out at the depyrogenation step. Products are washed, dried then passed through a heat tunnel to depyrogenate them at elevated temperatures (280-350 °C) to ensure they are sterile before being transferred directly to the filling operation.
In contrast, RTU container sterilization is carried out after final packaging, and prior to transportation to the aseptic fill and finish facility. In the subsequent process steps, outer packaging is removed and the sealed inner packaging is aseptically transferred into the filling operation. There are challenges in assuring sterility is maintained between the point of exit from the sterilization chamber to the point of entry into an aseptic isolator.
Succinctly put, sterility is all about probabilities, where the risk of surviving organisms on an object has to be minimized by validated processes and qualified packaging solutions. For RTU packaging, sterility needs to be assured from the point of sterilization until it reaches the aseptic isolator.
RTU primary packaging from SCHOTT Pharma
SCHOTT iQ® is a holistic platform to standardize ready-to-use (RTU) packaging components and improve the quality of RTU containers. With SCHOTT iQ®, development and delivery of high-quality injectable drugs becomes efficient and significantly improves the safety of patients.
Available variants within the SCHOTT iQ® platform are adaptiQ®, syriQ® and cartriQ®, which are all packed in standardized tubs.
validating-and-maintaining-sterility_accordion_FAQ-01
Sterility is quantified by the Sterility assurance Level (SAL), which indicates that a product has been rendered sterile. A SAL of 10-6, for example, is the levelrequired by the US FDA for medical devices and injections under the definition of an "overkill" approach. According to USP, "overkill" sterilization can be defined as a method in which the destruction of a high concentration of a resistant microorganism supports the destruction of reasonably anticipated bioburden present in routine processing.
The SAL is determined by challenging the sterilization cycle with high levels of resistant organisms and quantifying the number of any surviving colony forming units (CFUs) after pharmaceutical product sterilization.

Dr. Robert Lindner
Product Manager Bulk & Sterile Solutions