
Critical advantages of smart primary packaging containers
Track-and-trace solutions connected directly with drug containers have been on the pharmaceutical radar for quite some time. Recently, the International Society for Pharmaceutical Engineering (ISPE) published the “Good Practice Guide: Unique Identification of Glass Primary Containers in Pharmaceutical Fill & Finish Operations”, which delivered industry standards and recommendations for these solutions.
What is primary packaging?
Primary packaging refers to the material that comes into direct contact with a product. In various industries, this first layer of packaging is available in formats such as containers, covers, and wrappings. In the pharmaceutical industry, primary packaging is specifically designed to protect the drug product from contamination and ensure it remains safe during storage and transportation.Primary packaging options for the safe storage of injectable drugs include syringes, cartridges, vials, and ampoules. SCHOTT Pharma offers a range of primary packaging solutions made from glass and high-grade COC polymer.
Selecting the right primary packaging is crucial for ensuring patient safety. Manufacturers such as SCHOTT Pharma continuously innovate to enhance the safety and efficiency of drug containers throughout the entire supply chain. An example of such innovation is smart primary packaging containers, which help to prevent mix-ups during production and transportation.
What is smart primary packaging?
To enhance fill-and-finish processes in the pharmaceutical industry and pave the way towards a more effective, digitalized future in drug container manufacturing, SCHOTT Pharma has developed a line of smart primary packaging containers. These containers, known as SCHOTT Pharma Smart Containers, are laser-marked with a unique data matrix code during manufacturing to enable high levels of traceability throughout the fill-and-finish process. Manufacturers can monitor each container for several different factors, including retention time, temperature, and weight during infeed, depyrogenation, and filling.
Types of Container Unique Identifier
A Container Unique Identifier (CUID) is an individual combination of numeric or alphanumeric characters that is physically applied to the pharmaceutical drug container using a CUID data carrier. Two data carriers are mainly used for this purpose: a Data Matrix Code (DMC), which is a 2D barcode, and radio-frequency transponders (RFID).Five advantages of smart primary packaging containers
There are several key advantages to SCHOTT Pharma’s smart primary packaging containers, from streamlining the fill-and-finish process to utilising the latest developments in advanced technology. In this section, we will look at these advantages in more detail.
Reduce the risk of mix-ups
In the past few years, the production sites of a number of pharmaceutical companies have been audited by national drug associations due to mix-up events that have affected either the active components of the medication, the dosage, or the labeling of the final product. The risks are widespread if the drug is filled in different concentrations on the same site, or when different steps of the filling process are performed at different locations. For example, the vials are kept in storage after filling in one location and the secondary packaging is done in another.
A CUID read at the beginning of the fill-and-finish process ensures the pharmaceutical companies know what they are filling in each of the containers and the type of polymeric or elastomeric component that is added to it. It even allows the code to be linked with the bar code available on the secondary packaging. The container’s content can therefore be identified throughout the whole process.
Easy container-targeted recalls
There are a number of consequences of a drug being recalled from the market: it can put the patient’s health at risk if not done correctly and in time; it can cost a vast amount of money; and it can also damage the reputation of the pharmaceutical company manufacturing the medication.
If every single container carries a data matrix code linked to the barcode on the secondary packaging, traceability can be achieved until the last steps of the supply chain – private pharmacies, clinics, hospital inventory, etc. In this case, when the affected batches have been identified, the individual products can be recalled from the market, reducing the impact of the recall for all stakeholders.
Corrected reject management
During the fill-and-finish processing of pharmaceutical containers, vials, syringes, cartridges and ampoules can be rejected at different stages (for example, washing, filling, and closing), either because the containers are defective or incorrect parameters have been used on the line.
If smart packaging solutions are used and read at different stages of the filling process, rejects can be linked to specific process steps and corrective action can be taken.
Improved line clearance
After each batch production is complete, the fill-and-finish line needs to be checked so that no production material (container, polymer components, etc.) remains. If this is not done properly, a vial intended for a certain filling volume or a cap whose color is linked with a specific drug product could be used incorrectly, constituting a risk to patient health.
Being able to read containers and other components at multiple stages makes the identification of spots where they went missing easier, simplifying the manual task of line clearance.
Optimization of the lyophilization process
The number of drug medications in vials that need to be lyophilized to keep them stable and retain their properties is growing at a rapid pace. Since this is a very complex, time-consuming processing step for pharmaceutical companies and CDMOs, every improvement is a benefit.With CUID implemented on each container, being able to trace the position of each vial will optimize the lyophilization cycle. As the exact position of each vial in the chamber can be determined, zones can be identified where the lyophilization process is not working and defects such as fogging and lyo-cake collapsing are occurring. This simplifies the application of corrective action when required.
EVERIC® smart & syriQ® smart. SCHOTT Pharma Track & Trace solutions
SCHOTT Pharma’s product portfolio currently includes two types of glass containers with a data matrix code implemented through gentle laser marking.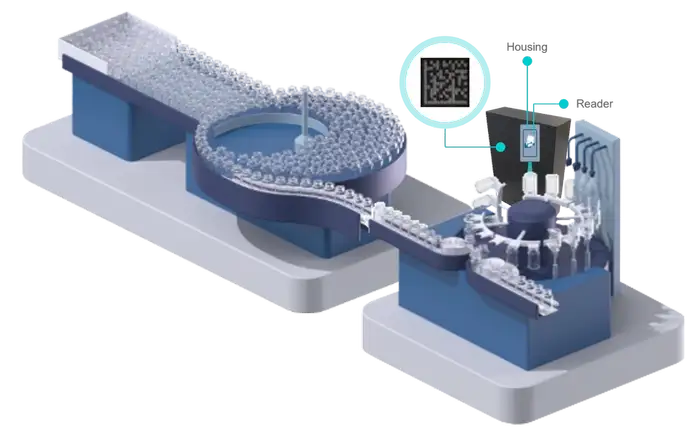


The CUID code applied on both types of the smart packaging consists of up to 44 respectively 31 randomized numeric respectively alphanumeric digits. Thanks to unlimited possible combinations, a single code’s combination will not repeat itself for several years. In addition, while reading the code during the fill-and-finish process, any type of data relevant to the drug manufacturer can be added via a data management system.
SCHOTT Pharma chose laser marking to apply the code for a number of reasons:
- The code is inextricably linked to the container during the manufacturing process.
- Since the code is made from existing glass, it is not removable without leaving marks. In addition, no material is added that might require additional registration or poses the risk of introducing foreign particles.
- Using the latest state-of-the art laser technology ensures a low impact on the glass structure, maintaining the container’s strength throughout the entire fill-and-finish process. The highest accuracy and consistency is achieved by employing this technology rather than, for example, ink.
- The speed of the line is not limited due to the reading of the code. In the case of vials, a readability up to 1,000 items per minute has been demonstrated.
SCHOTT Pharma’s smart packaging technology provides the basis for the pharmaceutical industry to take the next step towards track-and-trace, offering multiple benefits that improve the fill-and-finish process, reduce the total cost of ownership, and enhance patient safety.

Victoria de la Torre
Global Product Manager ampoules