Redefining safety, efficiency, and sustainability: SCHOTT Pharma launches next generation polymer syringe system
Thursday, January 16, 2025, Mainz, Germany
- A new cap design for prefillable polymer syringes provides tamper evidence at the individual syringe level, significantly reducing the risk of drug waste and ensuring patient safety.
- The integrated label concept, developed with Schreiner MediPharm, offers reliable first-opening indication, oxygen and light protection, and can include an RFID chip for accurate drug traceability and inventory management.
- A carton secondary packaging eliminates the need for blister packs, reducing waste and saving valuable storage space in hospitals and during transport, thereby cutting the carbon footprint of the entire value chain by up to 58%.
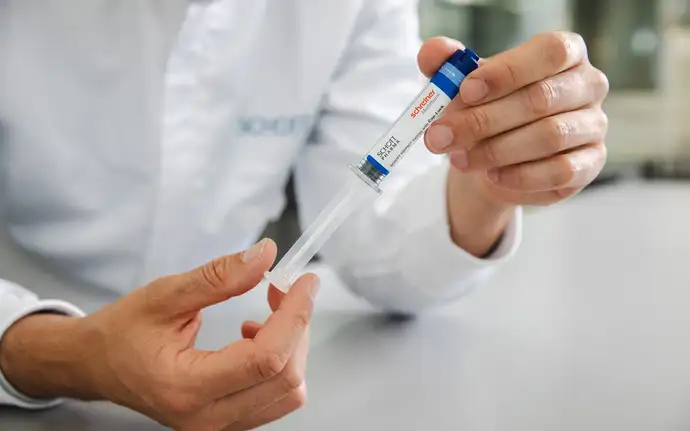
At the heart of the new syringe system is a pre-assembled cap that simplifies the filling process for pharmaceutical companies. The next generation of SCHOTT TOPPAC® infuse syringes can be filled on existing filling lines without requiring modifications or additional investment. Combined with a functional label, the new design covers the syringe shoulder completely, providing a first-opening indication for each syringe. With conventional syringe systems, the first-opening mark can only be found on the secondary packaging. However, when provided directly at the point of use, the syringe can be re-used if it has not been previously opened. Combined with the high-quality cyclic olefin copolymer (COC) material used to manufacture SCHOTT TOPPAC® infuse, the new syringe system ensures drug stability throughout its shelf life, thus reducing the risk of drug contamination.
The new cap design allows for a functional label that provides additional protection, such as oxygen and light protection, but can also contain relevant information about the specific medication, such as expiration date or batch number. These can be digitalized by integrating them into the label with an RFID chip, enabling an automated, more efficient medication inventory process. "An RFID chip in the label reduces the preparation time of an anesthesia tray, for example, from 20 minutes to just two minutes, significantly increasing operational efficiency. Moreover, the label-integrated RFID chip enables a digital first-opening indication," explains Dr. Nadine Lampka, Senior Product Manager Pharma-Security at Schreiner MediPharm.
While traditional secondary packaging for prefilled polymer syringes consists of a polymer blister, the new cap and label features provide the basis for a blister-free package, while allowing for a first-opening indication, maintaining the integrity of the prefilled syringe and protecting against mechanical impact or dust. This allows the use of cartons instead of polymer blisters, which are easier to open for nurses and doctors, less space-consuming for disposal, and easier to recycle.
Reduced time to market
The new SCHOTT TOPPAC® infuse prefillable polymer syringe system also accelerates time-to-market for pharmaceutical companies. SCHOTT Pharma's expertise in regulatory and analytical testing accelerates product development and enables pharmaceutical companies to meet industry requirements with confidence. In addition, they can continue to use existing data collected with the syringe, as only the closure has changed, simplifying the transition process.Available in 3, 5, and 10ml volumes, the next generation of SCHOTT TOPPAC® infuse polymer syringes are set to revolutionize the healthcare industry by providing a more efficient, stable, and sustainable solution for drug delivery.

The new system for prefillable polymer syringes from SCHOTT Pharma uses a carton secondary packaging instead of blister, reducing waste and CO2 emissions along the value chain. Photo: SCHOTT Pharma/Oana Szekely
About SCHOTT Pharma
Human health matters. That is why SCHOTT Pharma designs solutions grounded in science to ensure that medications are safe and easy to use for people around the world. The portfolio comprises drug containment solutions and delivery systems for injectable drugs ranging from prefillable glass and polymer syringes to cartridges, vials, and ampoules. Every day, a team of around 4,700 people from over 60 nations works at SCHOTT Pharma to contribute to global healthcare. The company is represented in all main pharmaceutical hubs with 16 manufacturing sites in Europe, North and South America, and Asia. With over 1,000 patents and technologies developed in-house and a state-of-the-art R&D center in Switzerland, the company is focused on developing innovations for the future. SCHOTT Pharma AG & Co. KGaA is headquartered in Mainz, Germany and listed on the Frankfurt Stock Exchange as part of the MDAX. It is part of SCHOTT AG, which is owned by the Carl Zeiss Foundation. In light of this spirit, SCHOTT Pharma is committed to sustainable development for society and the environment and has the strategic goal of becoming climate-neutral by 2030. Currently, SCHOTT Pharma has over 1,800 customers including the top 30 leading pharma manufacturers for injectable drugs and generated revenue of EUR 957 million in the fiscal year 2024. Further information at www.schott-pharma.com

Lea Kaiser
PR & Communications Manager
 Research Gate
Research Gate