
Pharmaceutical product design that is safe for the patient and sustainable for the planet
Did you know that most of a product's environmental impact is already determined during development? Therefore, at SCHOTT Pharma, we have implemented Ecodesign to systematically mitigate what matters the most where it matters the most, allowing us to create products that are safe for the patient and sustainable for the planet.
To really make a difference, we must tackle the problem at its roots
Products have an environmental impact throughout their lives, from production to disposal. However, 80% of a product's environmental impact is determined in the design stage. That is according to figures from the Ellen MacArthur Foundation, a global leader in the field of Sustainability. Marielle Girard, a product engineer at SCHOTT Pharma, notes: “This is why decisions in the product development phase can make or break our sustainability aspirations.”
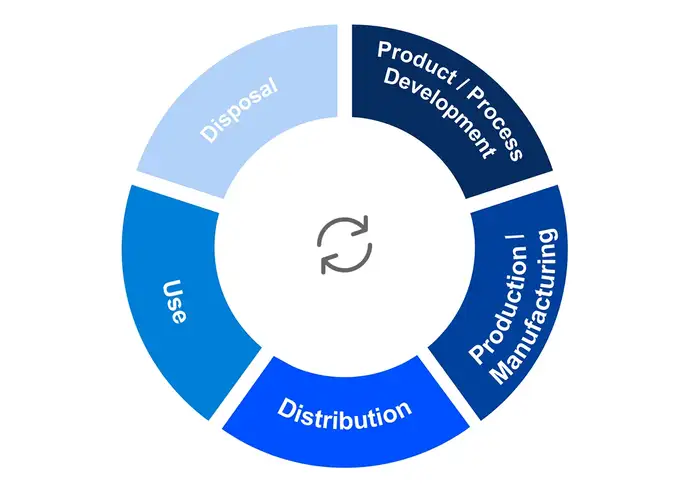
Visualisation of a typical product lifecycle
Ecodesign is our best bet to decrease our environmental impact
Ecodesign is a development approach that can effectively reduce the environmental impact of products. Formally, Ecodesign is “the integration of environmental aspects into the product development process by balancing ecological and economic requirements” (European Environment Agency, 2017). In practice, Ecodesign at SCHOTT Pharma implies that company-specific Dos and Don’ts are considered in product development. For instance, by maximizing packaging density, packaging material can be reduced. Additionally, by increasing the number of containers per packaging unit, downstream processes, such as sterilization or transportation, become more efficient. “At SCHOTT Pharma, everything we know about Ecodesign from different parts of the organization, customers, and partners is aggregated, sorted, and continuously updated in a central document, our Ecodesign Guideline. Thereby, we guarantee that comprehensive, state-of-the-art Ecodesign knowledge is readily available to product development.” says Anna.
By making Ecodesign an integrated element in our product development process, we walk the talk
“Applying the basic principles underlying Ecodesign has always been common practice at SCHOTT Pharma,” says Marielle. “However, by formalizing Ecodesign and making it a mandatory element of the product development process, SCHOTT Pharma has institutionalized Ecodesign.”

To ensure design compliance with user requirements, all product developments at SCHOTT Pharma follow the principles of design control, with regular design reviews at stage gates. Ecodesign review is integrated into this proven development process that has put SCHOTT Pharma at the forefront of patient safety. The Ecodesign checklist must be filled out and reviewed by product engineers in every design review. By including the most critical Ecodesign principles, the Ecodesign checklist ensures that Ecodesign is anchored in everyone’s thinking and considered in every process from the beginning. Thereby, the Ecodesign checklist, combined with institutionalized design reviews, ensures that design sins are precluded from the start, and Ecodesign can be systematically improved from stage gate to stage gate. “At SCHOTT Pharma, we believe that integrating Ecodesign in the development process and providing regular training sessions to our product engineers puts us on the right track to develop products that are both safe for the patient and sustainable for the plant,” Anna adds.
You would like to know more?
Contact a member of the SCHOTT Pharma sustainability team today. We are open to collaboration and look forward to making an impact with you.

Anna Mader
Sustainability Professional
Register for the latest news
Stay up-to-date with information about SCHOTT Pharma products and services and register for our newsletter.