
5 steps for particle reduction in RTU packaging
Particle control is essential for the safety of pharmaceutical products and compliance with EU GMP Annex I requirements, which will become effective in August 2023. Failure to control particles can compromise product quality, efficacy, and patient safety, and may lead to regulatory non-compliance. Therefore, it is necessary to implement effective measures such as cleanroom requirements, particle reduction and monitoring, primary packaging inspection, process equipment validation, and personnel hygiene to ensure that pharmaceutical products meet the required standards and are safe for patients.
Particles can come from a variety of sources, including the container itself, the stopper, the drug formulation, and the environment. They are classified based on their size:
- Visible particles typically are larger than 150 μm (dependent on, e.g., particle shape and contrast)
- Sub-visible particles are smaller and cannot be detected without the aid of technical devices.
Controlling the particle levels in pharmaceutical containers is essential to ensure product quality and patient safety. Regulatory bodies such as the United States Pharmacopeia (USP) have developed standards to control the particle levels in primary packaging containers to ensure the quality of pharmaceutical containers. These standards are outlined in several USP chapters, including USP<790>, USP<787>, USP<788>, and USP<789>.
Nevertheless, data shows that particulate contamination was the leading cause of drug recalls between 2017 and 2021, contributing to 30% of all parenteral drug-related recalls (Source: FDA). Further research has identified primary packaging (containers, closures, etc.) as the leading source of visible particles in drug products, as glass-to-glass contact can lead to breakages and defects, which generate particles that infiltrate the product.
Manufacturers have attempted to resolve this issue through ready-to-use (RTU) components delivered in a nested configuration to reduce particle-generating defects. However, particulate matter in injectable drug products remains a significant concern, and it is evident that pharmaceutical developers must establish particle control measures in RTU packaging to ensure the safety and availability of essential medications. In this article, we will highlight five steps that help you reduce particle contamination in RTU packaging - for in-depth details, download the whitepaper below.
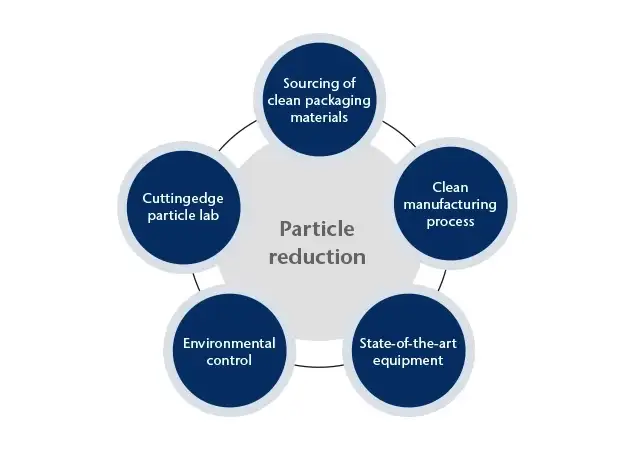
1. Sourcing of clean packaging materials
The first step in any particle reduction program for RTU containers, e.g., vials, is sourcing high-quality packaging materials free from impurities. This includes glass vials manufactured in a controlled environment using stringent quality control processes. By selecting packaging materials from industry-leading, qualified suppliers, manufacturers can ensure their products have exceptionally low levels of defects and contaminants.
2. Particle reduction through clean manufacturing process
The manufacturing process itself plays a critical role in reducing particulate matter in injectiable drug products. This includes ensuring that all equipment is properly cleaned and maintained and that staff adhere to strict hygiene protocols. To minimize the risk of contamination, it is essential to use cleanroom technology, including isolators or RABS (Restricted Access Barrier Systems), to mitigate the proliferation of particles and other contaminants.
3. State-of-the-art equipment
State-of-the-art equipment is essential for reducing particle contamination. This includes automated filling and packaging machines designed to minimize the risk of particle generation. Manufacturers can ensure that their products meet the highest quality and safety standards by using equipment specifically designed for RTU components.
4. Environmental control
Environmental control is critical for ensuring the manufacturing environment is free from particles and other contaminants. This includes maintaining a positive pressure environment to prevent the infiltration of particles from outside and using HEPA (High-Efficiency Particulate Air) filters to remove particles from the air. Additionally, it is important to monitor the environment regularly to ensure that particle levels remain within acceptable limits.
5. Cutting-edge particle lab
A cutting-edge particle lab can provide invaluable insights into the quality of RTU components. Advanced analytical techniques, such as laser diffraction, microscopy, and chromatography, can identify and quantify particles in packaging materials. This can help manufacturers identify potential contamination sources and develop targeted strategies for reducing particle levels.
Ensure patient safety with adaptiQ®
Particle contamination is a significant concern for pharmaceutical companies, as it can compromise patient safety and lead to costly recalls. By implementing this holistic approach to reducing particle contamination in RTU packaging, manufacturers can ensure that their products meet the highest quality and safety standards.

If you would like to learn more, download our whitepaper “Winning combination: Reducing particles in RTU packaging” or contact us.
Frequently asked Questions
In terms of pharma, particulate contamination refers to the solid particulate matter that can be found in almost all injectable drug products. The contamination mainly occurs during the manufacturing process, and it includes, for instance, dust, rubber, silicone and fibers. As particulate contamination may cause harm to patients, the goal is to minimize the particulate matter as much as possible to avoid risks and potential consequences.

Dr. Robert Lindner
Product Manager Bulk & Sterile Solutions
Register for the latest news
Stay up to date with information about SCHOTT Pharma products and services by registering for our newsletter.