
How to optimize fill-and-finish processes with sterile drug containment solutions
The pharmaceutical industry is constantly seeking ways to optimize manufacturing processes to increase efficiency, reduce costs, and maintain the highest standards of quality and safety. Sterile ready-to-use packaging solutions have emerged as a critical component in streamlining fill-and-finish processes, leading to significant improvements in product quality, patient safety, and time-to-market. This article explores RTU packaging solutions' three main value drivers and how they can transform the fill-and-finish landscape for pharmaceutical manufacturers.
Focus on the core: Reducing value chain complexity by outsourcing production steps
Pharmaceutical companies increasingly use outsourcing strategies to simplify their manufacturing processes and streamline their value chain. By shifting from traditional bulk drug containment solutions to sterile RTU packaging, companies can reduce the total cost of ownership (TCO) and focus on their core competencies.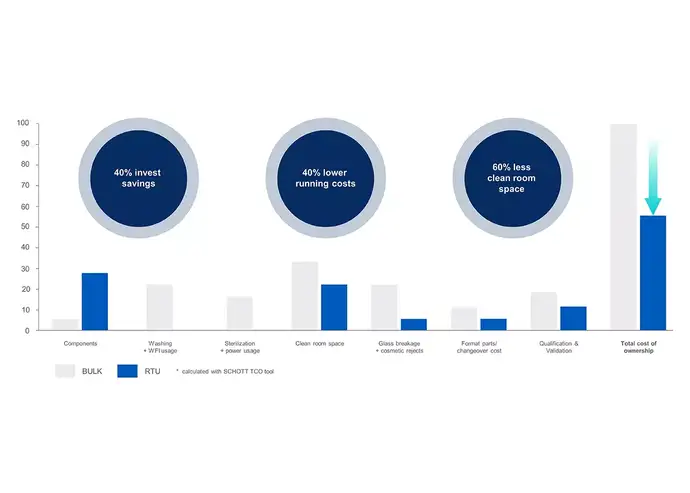
> Learn more about how RTU glass vials can reduce the Total Cost of Ownership
Ensure patient safety: Detecting and eliminating areas of risk along the value chain
Ensuring particle control is crucial for maintaining the safety of pharmaceutical products and adhering to the EU GMP Annex I guidelines, which will be enforced starting in August 2023. Neglecting proper particle control can jeopardize the quality, effectiveness, and safety of the products, resulting in non-compliance with regulatory standards and costly recalls.By implementing sterile RTU packaging solutions, pharmaceutical manufacturers can significantly reduce the risk of particle contamination. RTU glass vials arrive at the manufacturer washed, depyrogenated, and sterilized, ready for filling operations. This minimizes the risk of contamination and ensures a higher degree of automation, which in turn reduces labor costs and enhances overall product quality. Moreover, RTU packaging solutions facilitate a more standardized and efficient approach to particle reduction, ensuring patient safety throughout the value chain.
However, particle contamination remains a significant concern, and it is evident that primary packaging suppliers must establish particle control measures in RTU packaging to ensure the safety and availability of essential medications.
> Discover 5 steps how RTU packaging reduces particle contamination
Gain speed: Maximizing patent lifetime by entering into the market earlier
In today's competitive pharmaceutical landscape, accelerating time-to-market is critical for maximizing patent lifetime and optimizing return on investment. When time-to-market (TTM) is key, three key aspects must be considered: Standardized packaging, quick changeovers, and machine compatibility.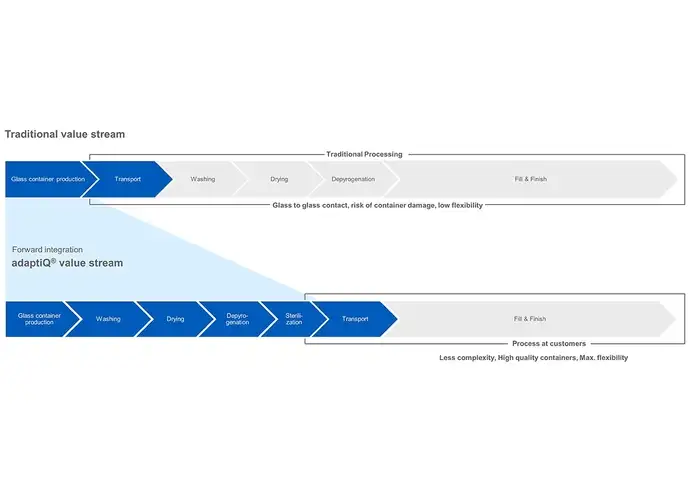
> Find out how RTU packaging can accelerate time-to-market
Optimize your fill-and-finish processes now
By embracing sterile RTU packaging solutions, pharmaceutical manufacturers can optimize their fill-and-finish processes and unlock significant benefits in value chain simplification, patient safety, and time-to-market. As the industry continues to face increasing challenges and regulatory demands, the adoption of RTU packaging solutions will be crucial for maintaining a competitive edge and ensuring the highest standards of quality in drug products.If you would like to learn more, look at our solution page “Improve your manufacturing processes with sterile drug containment solutions”. Let’s rethink your processes together.

Dr. Robert Lindner
Product Manager Bulk & Sterile Solutions
Register for the latest news
Stay up to date with information about SCHOTT Pharma products and services by registering for our newsletter.